how to find core electrons|6.4 Electronic Structure of Atoms (Electron Configurations) : Cebu The chemical reactivity of an atom is mainly determined by valence electrons. Atoms which have a complete shell of valence electrons tend to be . Tingnan ang higit pa cashback. Online Casino 🤑 Best Games - Play at 1xBet casino online 1xBet first deposit bonus ᐉ 1xbet.global.
PH0 · ⚗️ Identify Valence Electrons and Core Electrons
PH1 · What are Core Electrons?
PH2 · Khan Academy
PH3 · How do you find core and valence electrons? + Example
PH4 · Core electron
PH5 · CHEMISTRY 101: Valence and core electrons
PH6 · 6.4 Electronic Structure of Atoms (Electron Configurations)
PH7 · 3.4: Core and Valence Electrons
PH8 · 3.1: Electron Configurations
PH9 · 1.9B: Valence and Core Electrons
Latest lotto draw results for March 18, 2024: PCSO 6/45 mega lotto, Lotto 6/42, 6/49 super lotto, 6/55 grand lotto 6/58 ultra lotto, lucky pick in the Philippines. We use cookies to ensure you get the best browsing experience.
how to find core electrons*******The electrons of an atom are typically divided into two categories: valence and core electrons. Valence electrons occupy the outermost shell or highest energy level of an atom while core electrons are those occupying the innermost shell or lowest energy levels. Tingnan ang higit pa
how to find core electronsIn this module, the conceptions of valence and core electrons are put forward and explained in the introduction. The general rule of relationship between . Tingnan ang higit pa6.4 Electronic Structure of Atoms (Electron Configurations)In this module, the conceptions of valence and core electrons are put forward and explained in the introduction. The general rule of relationship between . Tingnan ang higit paThe chemical reactivity of an atom is mainly determined by valence electrons. Atoms which have a complete shell of valence electrons tend to be . Tingnan ang higit paMiessler, Gary L., and Donald A. Tarr. Inorganic Chemistry. Upper Saddle River, NJ: Pearson Prentice Hall, 2010. Print. Brown, Ian David. The . Tingnan ang higit paAgo 21, 2017 — Learning Objective: Evaluate the number of valence electrons and core electrons from the electron configurations.Topics: core electrons, valence electrons.Okt 1, 2018 — Since germanium is a main-group element, its valence electrons are those in the outermost principal energy level. For germanium, the n = 1, 2, and 3 principal levels are complete (or full) and.
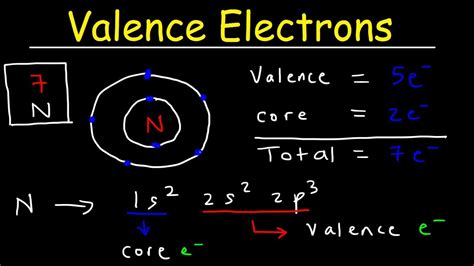
Hul 17, 2023 — We can see from the electron configuration of a carbon atom—1s 2 2s 2 2p 2 —that it has 4 valence electrons (2s 2 2p 2) and 2 core electrons (1s 2). You will see in .Core charge can be calculated by taking the number of protons in the nucleus minus the number of core electrons, also called inner shell electrons, and is always a positive .how to find core electrons 6.4 Electronic Structure of Atoms (Electron Configurations)The electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying the inner shell orbitals are called core .Since the core electron shells correspond to noble gas electron configurations, we can abbreviate electron configurations by writing the noble gas that matches the core electron configuration, along with the .Peb 15, 2023 — How to Find Core Electrons? To find the number of core electrons in an atom, you can use the periodic table to identify the element and then subtract the .If you're seeing this message, it means we're having trouble loading external resources on our website. If you're behind a web filter, please make sure that the domains .Peb 15, 2023 — 2. How to Find Core Electrons? To find the number of core electrons in an atom, you can use the periodic table to identify the element and then subtract the number of valence electrons from the total number of electrons for that element. The number of valence electrons is equal to the element’s group number on the periodic .The second shell (2s and 2p) has a total of 8 electrons. And, the third (last) shell has 1 electron. The first and second shells comprise the core (inner) electrons = 2 + 8 = 10 electrons. The outermost (valence) has 1 .
May 1, 2024 — Find your element on the table. Now, locate the element that you want to find the valence electrons for on the table. You can do this with its chemical symbol (the letters in each box), its atomic number (the .
In Figure 1B, if a 2p electron exists at a distance r 1, most likely the 1s electrons (core electrons) will be between the electron of interest and the nucleus. But, there is only a small probability of the 2 s electron (electron in the same valence shell) to shield the 2p electron of interest.
Abr 22, 2014 — About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features NFL Sunday Ticket Press Copyright .
Mar 6, 2023 — To find the number of electrons an atom has, start by looking up the element you're working with on the periodic table and locating its atomic number, which will be in the upper left-hand corner of the square. Then, identify the charge of the ion, which will be written as a superscript to the right of the element. .Electrons exist in orbitals around a nucleus.These orbitals and the energy needed to remove each of these electrons from the atom are set by quantum mechanics.Each of these orbitals serves to create a shell of electrons in the atom. Valence electrons are the electrons orbiting the nucleus in the outermost atomic shell of an atom. Electrons that .Each shell comprises subshells (s, p, d, f) accommodating a specific number of electrons. The outermost shell is called the valence shell and contains valence electrons. The remaining inner shells collectively contain the core electrons. Calculating Core Electrons. To calculate the number of core electrons in an atom, follow these steps: 1.
For sodium, [Ne]3s 1, the symbol [Ne] represents core electrons (1s 2 2s 2 2p 6) and the valence electron is the electron in the 3s orbital. Figure 1. A core-abbreviated electron configuration (right) replaces the core electrons with the noble gas symbol whose configuration matches the core electron configuration of the other element.Dis 15, 2019 — Learn how to identify an element from its successive ionization energies, a key concept in AP Chemistry, with this worked example video.
The atomic core is made up of an atom’s nucleus and core electrons. The nucleus is tightly connected to the core electrons. What number of core electrons does MG have? Mg has 10 core electrons and two valence electrons as a result of the 10 core electrons. The number of core electrons is determined by the element’s period (row). (equal to .

For our sodium example, the symbol [Ne] represents core electrons, (1s 2 2s 2 2p 6) and our abbreviated or condensed configuration is [Ne]3s 1. Figure \(\PageIndex{5}\): A core-abbreviated electron configuration .Set 20, 2022 — The electron being removed from a \(\ce{Mg}\) atom is a \(3s\) electron, which is only shielded by the inner core electrons. Since there is a greater degree of electron shielding in the \(\ce{Al}\) atom, it .Khanmigo is now free for all US educators! Plan lessons, develop exit tickets, and so much more with our AI teaching assistant.
Ago 14, 2020 — As electrons are removed from the outer valence shell, the remaining core electrons occupying smaller shells experience a greater effective nuclear charge Z eff (as discussed) and are drawn even closer to the nucleus. Figure \(\PageIndex{3}\): The radius for a cation is smaller than the parent atom (Al), due to the lost electrons; the radius .
If you're seeing this message, it means we're having trouble loading external resources on our website. If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.
Electron Shielding and Effective Nuclear Charge. If an electron is far from the nucleus (i.e., if the distance \(r\) between the nucleus and the electron is large), then at any given moment, many of the other electrons will be between that electron and the nucleus (Figure \(\PageIndex{1}\)). Hence the electrons will cancel a portion of the positive charge of the .Electron Shielding and Effective Nuclear Charge. If an electron is far from the nucleus (i.e., if the distance \(r\) between the nucleus and the electron is large), then at any given moment, many of the other electrons will be between that electron and the nucleus (Figure \(\PageIndex{1}\)). Hence the electrons will cancel a portion of the positive charge of the .
Key Totals Trends to Watch. Overs went 30-19 (61.2%) in Week 11. This brings the season-to-date over/under record to 262-274-4 (48.8%) as it continues to inch closer to 50%.; Week 11 saw a sharp .
how to find core electrons|6.4 Electronic Structure of Atoms (Electron Configurations)